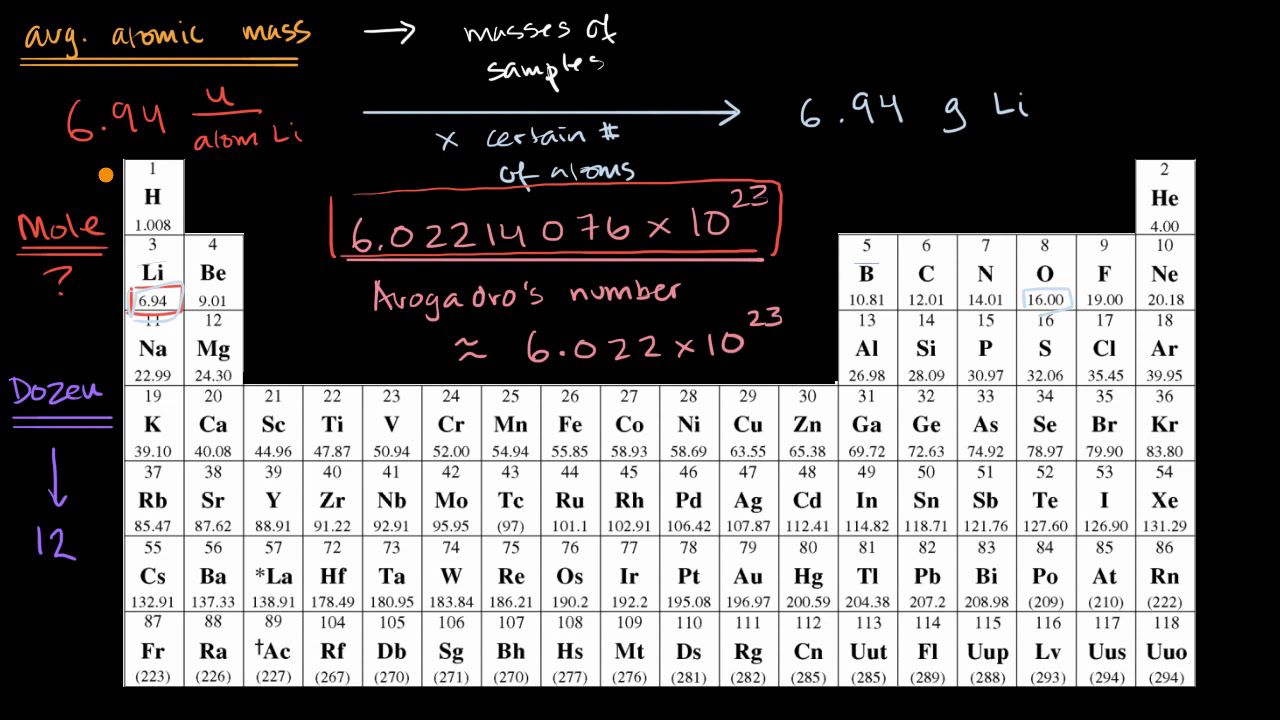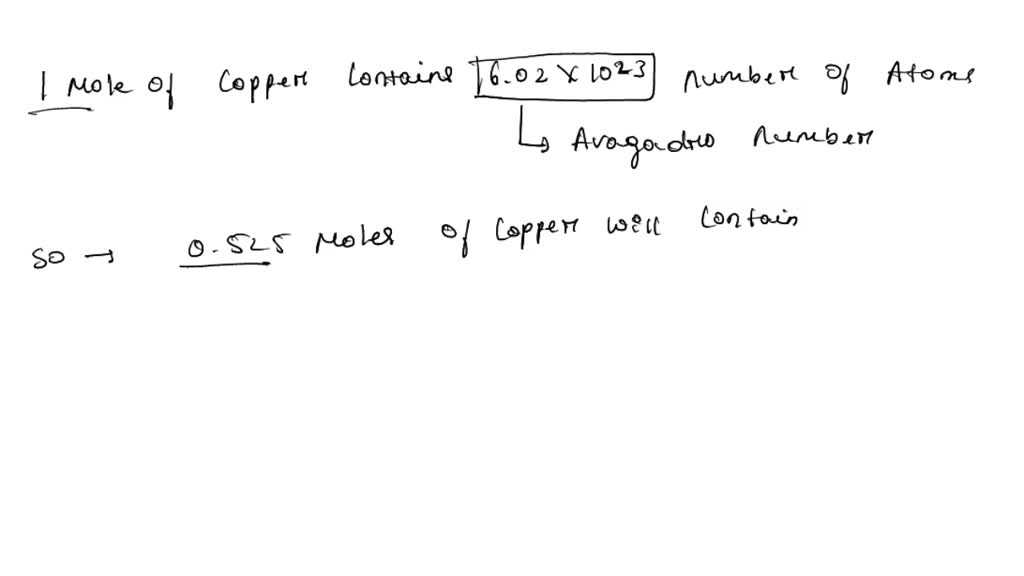
SOLVED: Knowing that Avogadro number is equal to 6.022 x 1023 atoms/mol, do the following: How many moles of iron are in 50.0 g of iron? How many moles of copper atoms

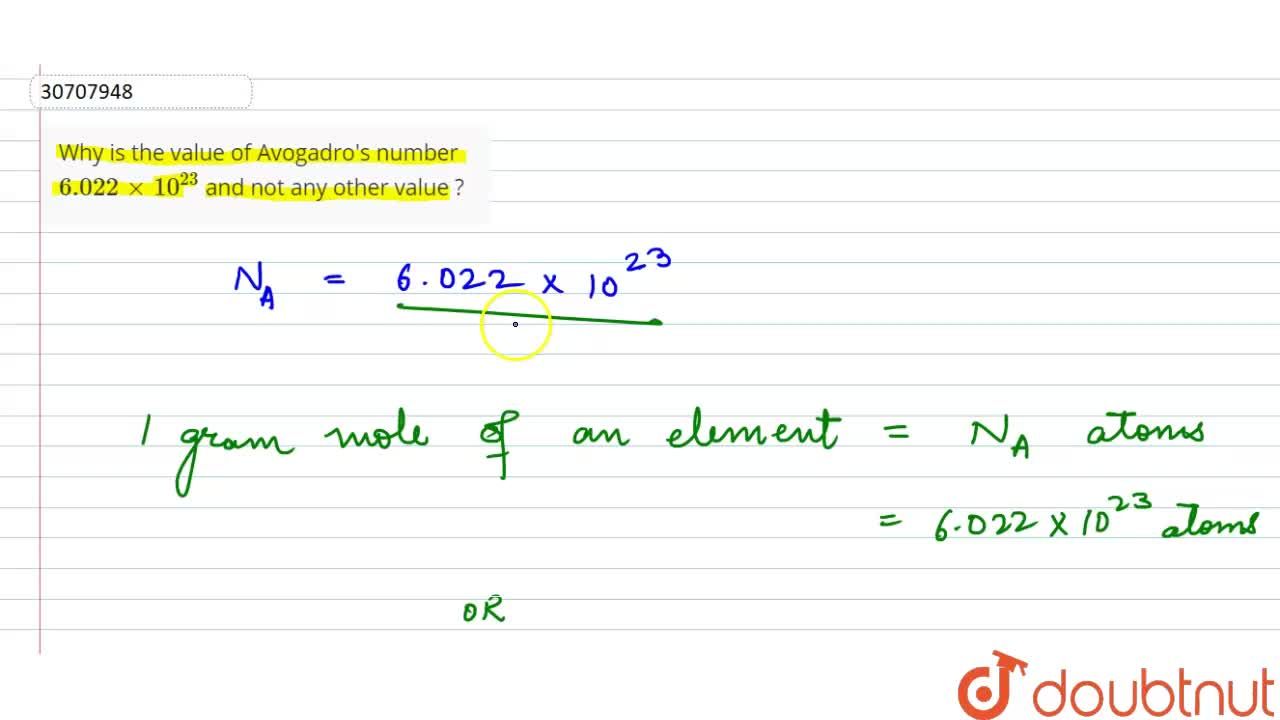
If Avogadro number `N_(A)` is changed from `6.022xx10^(23) mol^(-1)` to 6`.022xx10^(23) mol^(-1)`, - YouTube

12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)
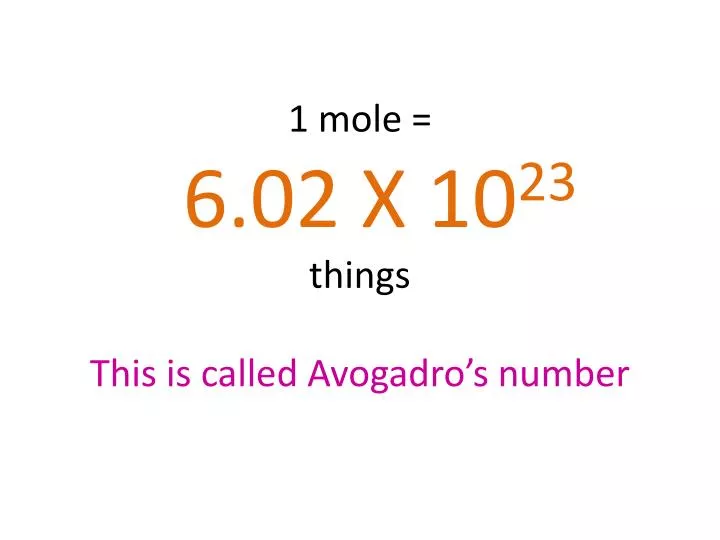
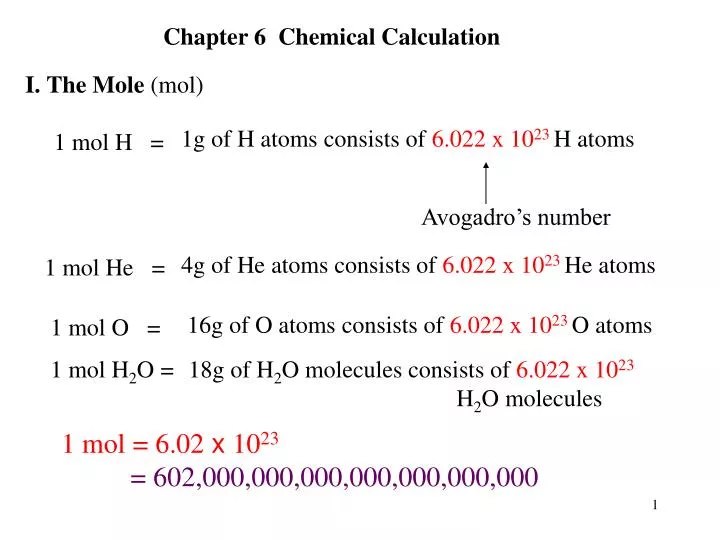
PPT - 1 mole = 6.02 X 10 23 things This is called Avogadro's number PowerPoint Presentation - ID:4272623

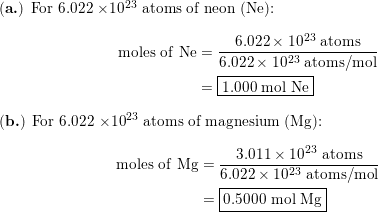
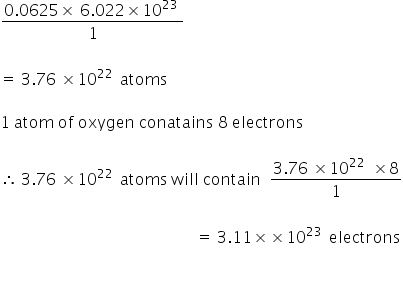


![How did Avogadro come up with the Avogadro number: [math]6.022 \times 10^{23}?[/math] - Quora How did Avogadro come up with the Avogadro number: [math]6.022 \times 10^{23}?[/math] - Quora](https://qph.cf2.quoracdn.net/main-qimg-ed17b8adf0c2f123329b850f1d223b9c.webp)